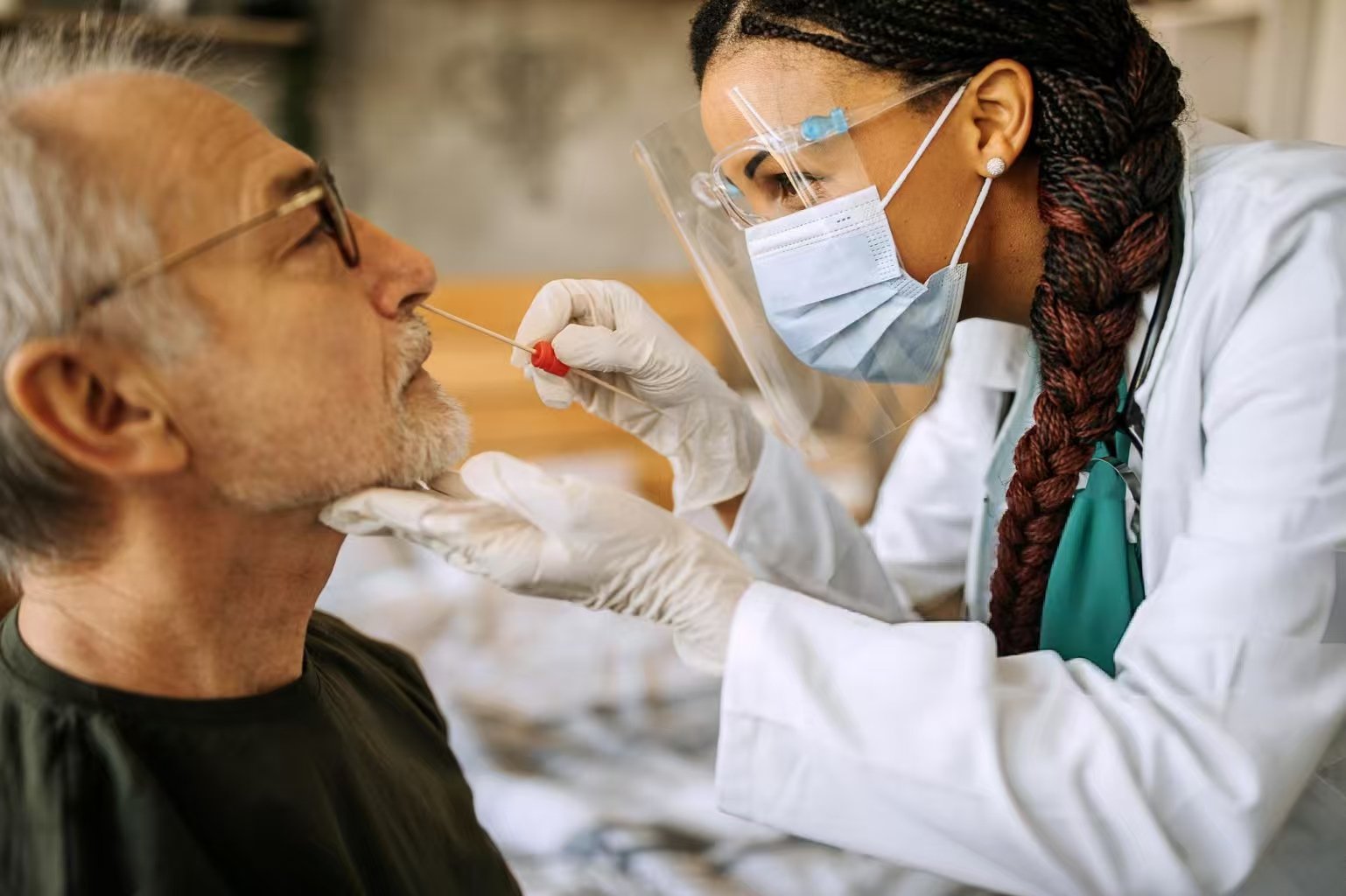
A new nasal vaccine for COVID-19, developed at Washington University in St. Louis, is set to begin Phase 1 clinical trials in the U.S. The U.S. Food and Drug Administration (FDA) approved the investigational new drug application from Ocugen, Inc., a biotechnology company that licensed the technology in 2022. This trial marks a significant step forward in fighting the virus.
1.Sponsorship and Trial Plans
The National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health (NIH), will sponsor and conduct the trial. The trial is set to begin this spring.
Although COVID-19 cases have dropped since the pandemic’s early days, the virus still spreads and causes illness. This new nasal vaccine is designed to strengthen immunity in the nose and respiratory tract, where the virus enters the body. This could stop the virus from spreading and reduce serious illness and death. Traditional vaccines are injected into the body, but they do not stop transmission.
2.Two Delivery Methods for the Vaccine
The trial will test the safety and effectiveness of two vaccine delivery methods: inhaling it into the lungs and spraying it into the nose.
3.Potential for Other Respiratory Infections
Doug E. Frantz, PhD, Vice Chancellor for Innovation at WashU, expressed excitement about this new vaccine technology. He believes it could help control COVID-19 and other respiratory infections like seasonal flu, avian flu, and respiratory syncytial virus (RSV).
A version of this nasal vaccine has been available in India since 2022 through a partnership with Bharat Biotech.
4.Phase 1 Trial Details
The Phase 1 trial will involve 80 adults between 18 and 64 years old. Participants will be divided into four groups: low-dose intranasal, high-dose intranasal, low-dose inhaled, and high-dose inhaled. The main goal is to test the vaccine’s safety. Researchers will also measure how well it boosts the immune system by checking antibody levels and tracking any breakthrough COVID-19 cases.
5.How the Vaccine Works
The nasal vaccine was developed by WashU scientists Michael S. Diamond, MD, PhD, and David T. Curiel, MD, PhD. They inserted a gene from SARS-CoV-2 (the virus that causes COVID-19) into a harmless adenovirus. This adenovirus then delivers the SARS-CoV-2 protein to the nose, helping the body build immunity without getting sick.
Diamond and Curiel’s studies at WashU showed that this vaccine generates a strong immune response, particularly in the nose and respiratory tract. Animal studies in 2020 and 2021 showed that the nasal vaccine prevented infection in the nose and lungs, potentially stopping the virus before it could cause harm.
6.Vaccine’s Impact on Virus Transmission
In 2021, another study showed that hamsters vaccinated with the nasal vaccine did not spread the virus to others, stopping transmission. Curiel noted that, while all vaccines help reduce sickness, nasal vaccines could also slow the spread of respiratory viruses, making them crucial in managing diseases like COVID-19, influenza, and others.
Source:WashU Medicine